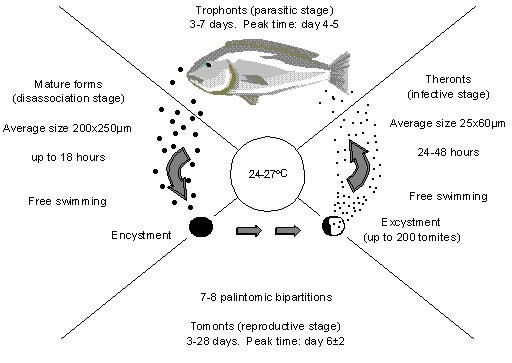
Figure 1: The life cycle of C. irritans (After Colorni, 1987).
Tuesday 17 June 2008
I recently answered some questions from a reader about Cryptocaryon and hyposalinity. I thought others might benefit from making my answers available so I have expanded on the answers I provided to the reader.
Here are the original questions:
My response was broken into three main areas, the variability of the duration of the phases of the life cycle; the efficacy of hyposalinity on the different stages; and the impact these have on treatment.
Figure 1 shows the stages in the life cycle of Cryptocaryon irritans.

Figure 1: The life cycle of C. irritans (After Colorni, 1987).
The problem with Cryptocaryon is that while the stages of the life cycle are well known and quite well understood, the duration of each stage is variable, especially the tomonts. This is partly varied with water temperature, but even with a constant temperature there is wide variation in the duration of the tomonts from as little as 3 days to 35 days (see Table 1). There is some information floating around from one of the researchers who claims to have seen viable tomonts that were still around after 2 months. I will take the last lot as an exception and assume 3 to 35 days at the temperatures we normally work: 24-27ºC.
Table 1: Lengths of stages of C. irritans from various studies.
| Reference | Trophont | Free-swimming Trophont | Tomont | Theront | Temperature |
| Burgess and Matthews, 1994a | 78 - 113 hrs | 2 - 8 hrs | 84 hrs - 35 days | up to 18 hrs | 23 - 27°C |
| Burgess and Matthews, 1994b | 78 - 113 hrs | 10 - 12 days | 24 - 26°C | ||
| Cheung et al., 1979 | 30 mins - 24+ hours | 8 - 9 days | 25°C | ||
| Cheung et al., 1979 | 30 mins - 24+ hours | 5 - 7 days | 30°C | ||
| Colorni, 1985 | 3 - 7 days | up to 18 hours | 3 - 28 days | 24 - 48 hours | 24 - 27°C |
| Diggles and Lester, 1996a | 3 - 7 days | 3 - 12 days | 25°C | ||
| Diggles and Lester, 1996b | 4 - 6 days | 3 - 15 days | 25°C | ||
| Yoshinaga and Dickerson, 1994 | 6 - 7 days | 4 - ? days | up to 12.5 hours | 23 - 25°C |
The next issue is the effectiveness of hyposalinity on the tomonts. Hyposalinity does not appear to be effective on the trophonts, perhaps because they gain some protection from the mucus of the fish. Colorni (1985) found that even after 18 hours in freshwater, trophonts attached to fish were still viable. Cheung et al., (1979) found that hyposalinity disrupted the divisions of the tomonts. Colorni (1985) found that tomites (theronts) could not tolerate salinities below 25‰. These results suggest that hyposalinity will be most effective on the tomonts and tomites/theronts, with greater efficacy on the latter.
In the Cheung, et al., (1979) study, the authors already had the salinity lower at the time the cysts formed. Under those conditions the low salinity impacted the divisions and there were no viable tomites/theronts. It appears that the Colorni (1985) study did the same thing. I don't believe there have been any studies on the effects of hyposalinity when it is applied during the tomont phase - i.e. when the tomonts have already encysted.
There is a study by Hirazawa et al., (2003) where they looked at various treatments for killing cysts (tomonts) and theronts. They found different results when the treatment was applied from early (1-16 hours after encystment) versus later in the stage (72 - 87 hours). Almost all the treatments were less effective on the later stages. One of the treatments was freshwater. In the early stages, freshwater for 1 hour was partially effective and 100% effective after 24 hours. On the later stage tomonts, 12.5+/- 21.7% were still viable after 24 hours. This to me suggests that if you start hyposalinity after tomonts have already encysted, some may survive and produce theronts.
Given the variability the trophont stage (3-7 days on the fish), it is quite likely that at the time of the starting hyposalinity, at least some trophonts have already dropped off the fish and encysted. If hyposalinity is not effective (or not as effective) on these tomonts, they may be able to complete their divisions and produce theronts. As they may take 28 days (or even longer) to release their theronts, you would want to keep the hyposalinity going for at least that long. As discussed, hyposalinity is effective on the theronts, so leaving the fish in hyposalinity until you are sure all tomonts have completed divisions and released their theronts gives the best possible chance of beating the parasites. This requires maintaining hypsoalinity for at least 4 weeks, with 6 weeks giving more margin for error.
That covers treatment, now on to prophylactic use of hyposalinity. Cryptocaryon is not the only parasite that can infect new fish and is also not the most deadly. The danger with prophylactic use of hyposalinity is if the fish are infected with something else, e.g. Amyloodinium, you'll have to raise the salinity before you can start treatment - copper is more toxic to the fish at lower salinity because it is generally harder to control the pH. I would prefer to just quarantine the fish without treatment and be ready to treat them immediately and appropriately at the first signs of infection.
I hope this helps.
Burgess P.J. and Matthews R.A. 1994. A standardized method for the in vivo maintenance of Cryptocaryon irritans (Ciliophora) using the grey mullet Chelon labrosus as an experimental host. J Parasitol 80:288-292.
Burgess P.J. and Matthews R.A. 1994. Cryotocaryon irritans (Ciliophora): photoperiod and transmission in marine fish. Journal of the Marine Biological Association of the United Kingdom 74:535-542.
Cheung P.J., Nigrelli R.F. and Ruggieri G.D. 1979. Studies on cryptocaryoniasis in marine fish: effect of temperature and salinity on reproductive cycle of Cryptocaryon irritans Brown, 1951. J. Fish Dis. 2:93-97.
Colorni A. 1985. Aspects of the biology of Cryptocaryon irritans, and hyposalinity as a control measure in cultured gilt-head sea bream Sparus aurata. Dis. Aquat. Org. 1:19-22.
Colorni A. 1987. Biology of Cryptocaryon irritans and strategies for its control. Aquaculture 67(1-2):236-237.
Diggles B.K. and Lester R.J. 1996. Influence of temperature and host species on the development of Cryptocaryon irritans. J Parasitol 82:45-51.
Diggles B.K. and Lester R.J. 1996. Variation in the development of two isolates of Cryptocaryon irritans. J Parasitol 82:384-388.
Yoshinaga T. and Dickerson H.W. 1994. Laboratory propagation of Cryptocaryon irritans Brown, 1951 on saltwater-adapted black mollies Poecilia latipinna. J. Aquat. Anim. Health 6:197-201.