Hyposalinity has been shown to be effective in the treatment of Cryptocaryon irritans infections by interfering with tomont division and theront survival (Cheung et al. 1979) however, what effect does it have on the fish that are being treated? This article discusses some of the issues related to the effects of hyposalinity on fish.
What is hyposalinity?
Hyposalinity involves lowering the salinity of the water. For the treatment of C. irritans infections, the salinity is lowered from normal (around 35‰) to 12-14‰ and kept at that salinity for 4 weeks or so to ensure that all surviving tomonts have completed division. For more information on hyposalinity, see: Hyposalinity.
Some basic fish physiology
The majority of fish we keep in our aquaria are known as teleosts on bony fishes. This excludes sharks and rays which are known as elasmobranchs. Teleosts have concentrations of salts in their blood and internal tissues about one third that of normal seawater (Schmidt-Nielsen, 1975) and this pretty much holds for both freshwater and marine teleosts. Marine teleosts lose water from their permeable body surface, particularly their gills, to the environment through osmosis. (Osmosis is the flow of water through a semi-permeable membrane to a concentrated solution.) In order to counteract the loss, the fish must drink a lot of seawater, however, as the seawater contains salts at a higher concentration than their body fluids, the concentration of salts in the body tends to increase. The fish excrete the excess salts, mainly through their gills, and this is an active (requires energy) process (Schmidt-Nielsen, 1975). Figure 1 summarises the processes of ionic regulation.
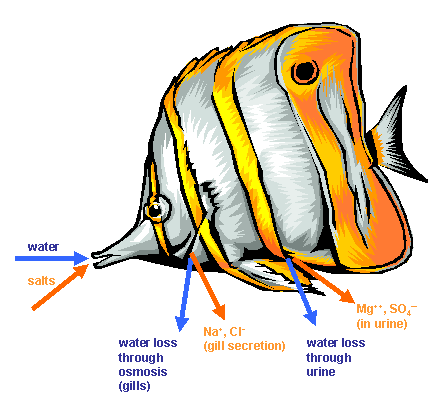
Figure 1: Ionic regulation in a marine teleost. The paths of water (blue arrows) and salts (orange arrows) is shown. The fish drink seawater (water and salts), but lose water through the gills and in urine. Salts are secreted by the gills and excreted in urine. (modified from Schmidt-Nielsen, 1975)
The tolerance of fish to changing salinity
It has long been assumed that most reef fish are stenohaline. This means they can only survive within a very narrow range of salinity and is based on the fact that reef habitats normally have a fairly constant salinity. Fish that live in estuaries and other brackish waters are considered euryhaline as they are able to tolerate a wide range of salinity. Research over the last few decades is shedding some doubt on the stenohaline status of many fish and it is possible that none or very few cannot tolerate variation in the salinity of their environment. Unfortunately, there hasn't many studies specifically on marine aquarium fish, but the studies into other species from the same or closely related families suggest that the fish we keep may have much higher tolerance than have previously been assumed.
A number of studies have found that numerous species of fish, including those found on coral reefs, are able to survive at salinities as low as 10‰ and lower with no apparent behavioural or physiological difficulties. Table 1 lists some species that have been studied. Of those studied to date, only one species, Carangoides equula, had difficulties around 10‰.
Table 1: Lower salinity limits for a number of species of marine teleosts.
| Species |
Common name |
Family |
Lower limit (‰) |
References |
| Plotosus lineatus |
Striped eel catfish |
Plotosidae |
5 - 10 |
Wu and Woo (1983) (as Plotosus anguillaris) |
| Epinephelus akaara |
Hong Kong grouper |
Serranidae |
3 - 5 |
Wu and Woo (1983) |
| Epinephelus awoara |
Yellow grouper |
Serranidae |
3 - 5 |
Wu and Woo (1983) |
| Terapon jarbua |
Jarbua terapon |
Terapontidae |
< 3 |
Wu and Woo (1983) |
| Carangoides equula |
Whitefin trevally |
Carangidae |
>10 |
Wu and Woo (1983) (as Caranx equula) |
| Lutjanus russellii |
Russell's snapper |
Lutjanidae |
< 3 |
Wu and Woo (1983) (as Lutjanus russelli) |
| Parapristipoma trilineatum |
Chicken grunt |
Haemulidae |
5 - 10 |
Wu and Woo (1983) |
| Pomadasys hasta |
|
Haemulidae |
5 - 10 |
Wu and Woo (1983) |
| Pagrus major |
Red seabream |
Sparidae |
5 - 10 |
Woo and Fung (1981); Wu and Woo (1983) (as Chrysophrys major) |
| Rhabdosargus sarba |
Goldlined seabream |
Sparidae |
5 - 10 |
Wu and Woo (1983) (as Rhabdosarga sarba) |
| Acanthopagrus schlegelii |
Black porgy |
Sparidae |
< 3 |
Wu and Woo (1983) (as Mylio macrocephalus) |
| Lethrinus nebulosus |
Spangled emperor |
Lethrinidae |
5 - 10 |
Wu and Woo (1983) |
| Pomacanthus imperator |
Emperor angelfish |
Pomacanthidae |
5 |
Woo and Chung (1995) |
| Siganus canaliculatus |
White-spotted spinefoot |
Siganidae |
3 - 5 |
Wu and Woo (1983) (as Siganus oramin) |
Anecdotal evidence from people using hyposalinity at 12-14‰ suggests that most bony fish kept in marine aquaria can tolerate this level of salinity.
Is hyposalinity beneficial to fish?
It has been suggested that hyposalinity reduces stress on fish and also reduces their energy requirements. The reasoning is that as maintaining an internal osmotic concentration less than that of their environment is an active process requiring energy, making the environment closer to the internal osmotic concentration will reduce the effort required for ionic regulation. Unfortunately, various studies into the effect of changing salinity on the metabolic rates, or at least oxygen consumption, have provided inconsistent results. It appears that not all fish react in the same way to variations in salinity. Nordlie (1978) proposed 4 different metabolic responses:
Oxygen consumption does not change significantly over a wide range of salinity.
Oxygen consumption is at its lowest when the osmotic concentration of the water is the same (isosmotic) as that of the fish's blood. Oxygen consumption increases if the salinity is increased or decreased from the isosmotic level and the increase is proportional to the concentration differences between the water and the blood.
Oxygen consumption is at its lowest when the fish is in its "normal" salinity and increases proportionally with changes (increases and decreases) in salinity.
Oxygen consumption is at its highest when the fish is in its "normal" salinity and decreases with changes (increases and decreases) in salinity.
References
Cheung P.J., Nigrelli R.F. and Ruggieri G.D. 1979. Studies on cryptocaryoniasis in marine fish: effect of temperature and salinity on reproductive cycle of Cryptocaryon irritans Brown, 1951. J. Fish Dis. 2:93-97.
Nordlie F.G. 1978. The influence of environmental salinity on respiratory oxygen demands in the euryhaline teleost, Ambassis interrupta Bleeker. Comp. Biochem. Physiol. A 59:271-274.
Schmidt-Nielsen K. 1975. Animal Physiology: Adaptation and Environment. Cambridge University Press, London. 699pp.
Woo N.Y.S. and Chung K.C. 1995. Tolerance of Pomacanthus imperator to hypoosmotic salinities: changes in body composition and hepatic enzyme activities. J. Fish Biol. 47(1):70-81.
Woo N.Y.S. and Fung A.C.Y. 1981. Studies on the biology of the red sea bream, Chrysophrys major. II. Salinity adaptation. Comp. Biochem. Physiol. A 69:237-242.
Wu R.S.S. and Woo N.Y.S. 1983. Tolerance of hypo-osmotic salinities in thirteen species of adult marine fish: implications for estuarine fish culture. Aquaculture 32:175-181.
Last updated: May 8, 2004
