 Figure 1: Density of pure water and seawater (S=35) over a range of temperatures (Based on equation in Pilson, 1998 for relating density to salinity).
Figure 1: Density of pure water and seawater (S=35) over a range of temperatures (Based on equation in Pilson, 1998 for relating density to salinity).
Specific gravity is used by aquarists to estimate the salinity of the water in their aquaria. Specific gravity is also used in a number of scientific disciplines, including chemistry, geology, biology and medicine. Unfortunately, specific gravity can be confusing and inconsistent as you will see from the following paragraphs. In order to understand specific gravity, it is important to also understand salinity.
What is salinity?
Salinity is essentially the saltiness of the water - how much salt is contained in the water. The definition has changed somewhat over the years because of the difficulty in measuring how much salt the water contains.
The intent was to be able to state the number of grams of salt (all the various solutes that make up seawater) in one kilogram of seawater. The main problem is that you can't just evaporate all the water leaving the pure salts that can be weighed. The heat required to dry all the salts will actually modify them, changing their mass and so introducing inaccuracies into the measurement.
It has been found that while the salinity of seawater may vary from location to location, the ratios of the major constituents change very little. Using this principle, it is possible to measure one of the major constituents and use that to determine the salinity. The concentration of chloride (plus bromide and iodide) can be determined by a titration with silver nitrate (Pilson, 1998) and this is known as the chlorinity. As the ratio of chlorinity to salinity is relatively constant, the latter can be determined from the former.
While the chlorinity is useful for determining the salinity of seawater, a titration is not always practical. In the latter half of the 20th Century electronic equipment became sensitive and accurate enough that the conductivity of seawater could be used to determine the salinity. This is now the primary means to determine salinity of seawater and the accepted definition of salinity (S) is defined in terms of the ratio of the conductivity of the sample to the conductivity of a standard solution of potassium chloride (KCl). It is also known as the practical salinity. Ideally, S is a unitless number, but many authors still use "psu" (practical salinity units), "ppt" (parts per thousand) or o/oo.
What is specific gravity?
Specific gravity is defined as the ratio of the density of a sample (at a specific temperature) to the density of some standard (at a specific temperature). For gases, the standard used is air. For most solids and liquids, including seawater, the standard used is pure water. The specific gravity of water in our tanks is therefore the ratio of the density of that water at a specific temperature to the density of pure water at a specific temperature. That sounds simple enough, but as we delve further it gets more complicated.
The density of a substance is the volume that a certain mass occupies. Or, to put it another way, it is the mass of a certain volume of the substance. Generally, as liquids and gases are heated they expand, and when they are cooled they contract. The density will therefore decrease with increasing temperature and increase with decreasing temperature. Pure water deviates from this slightly. Above 3.98°C, at normal atmospheric pressure, the relationship of temperature to density holds true, however, below 3.98°C, the density of pure water decreases and ice is even less dense that water at 0.0001°C. The maximum density of pure water is 1.000 g.cm-3 at 3.98°C. Figure 1 shows the density of pure water and seawater (S=35) over a range of temperatures.
 Figure 1: Density of pure water and seawater (S=35) over a range of temperatures (Based on equation in Pilson, 1998 for relating density to salinity).
Figure 1: Density of pure water and seawater (S=35) over a range of temperatures (Based on equation in Pilson, 1998 for relating density to salinity).
So, if temperature affects density, does it also affect specific gravity? Very much so. Not only is temperature part of the definition of specific gravity, but it also affects the measurement of it (which we shall see later). There are a number of standard temperatures for use with specific gravity and ideally any recording of specific gravity should indicate the temperatures. One such standard is to have both the sample and the pure water at 3.98°C. As the density of pure water is 1.000g.cm-3, the specific gravity of the sample using this standard will be equal to its density (in g.cm-3) at 3.98°C. Other standards include 20°C and 15.6°C (60°F). In most cases, the sample and pure water are measured at the same temperature, but this is not always the case. One common standard is 20°C for the sample and 3.98°C for pure water. The standard is usually written showing both temperatures, for example, 3.98°C/3.98°C (which is sometimes rounded up to 4°C/4°C) or 3.98°C/20°C. These are often abbreviated by dropping off the temperature unit as °C is assumed. For example, 20/20 or 20/4.
Specific gravity is a unitless measure. This is because it is a ratio of two densities and the units cancel themselves out. As specific gravity does not have any units, it will always be the same (for given temperatures) regardless of the units used for density. For example, for samples and pure water densities measured in kg.m-3, if the density of the sample is
How is specific gravity measured?
As specific gravity is essentially the density of a sample versus some standard, most devices for measuring the specific gravity are based on measuring the density of the sample. The most common method of measuring density is with a hydrometer. Hydrometers float in the sample and the height to which they float is governed by the density of the sample. The more dense the sample, the higher hydrometer will float. This phenomenon is described by Archimedes' Principle. Archimedes discovered (in that famous "Eureka!" bathtub scene) that a floating object will displace the volume of liquid with a mass equal to the total mass of the floating object as shown in Figure 2.
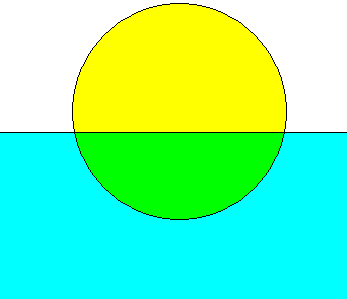
Figure 2: Archimedes' Principle. The yellow ball is floating on the blue water. The green represents the volume of liquid that is being displaced by the ball. The mass of that volume of liquid is equal to the mass of the whole ball.
If the mass of the floating object is known and there is some way to determine the volume of the floating object that is under the liquid (i.e. that has displaced that volume of liquid), the density of the liquid can be easily determined as the mass divided by the volume. This is exactly how a floating glass hydrometer works. The density of the sample, seawater in our case, influences the volume of the hydrometer under the water. The graduations on the stem of the hydrometer indicate the volume of the hydrometer under the water and therefore the density of the water. The graduations are calibrated so that they indicate the specific gravity.
As temperature affects the density of the sample being tested, it is important that temperature of the sample is the same as that for which the hydrometer has been calibrated. If the temperature of the sample is different from the calibration temperature of the hydrometer, the reading will need to be corrected to determine the true specific gravity. Good quality hydrometers usually come with tables for correcting the readings.
It is possible for the hydrometer to be calibrated for a different temperature than the standard to which it is measuring. For example, a hydrometer might measure the specific gravity using a standard of 20°C/3.98°C but maybe calibrated such that it gives the correct specific gravity for samples at 25°C. If the sample temperature is greater than 25°C, the reading will be lower than the correct specific gravity. If the sample temperature is lower than 25°C, the reading will be high.
Another form of hydrometer common in the marine aquarium hobby is the swinging arm hydrometers from Aquarium Systems, Coralife and other manufacturers. While the action of these hydrometers is quite different to the floating glass hydrometers, the principle is the same. The density of the sample influences the height at which the arm floats.
A refractometer also measures the density of the sample, but uses a very different property. The speed of light varies with the density of the medium through which it is travelling. The less dense the medium, the faster light travels. It travels at its fastest in a vacuum. If light passes from a medium of one density to a medium of another density at an angle, the rays of light are refracted. This is most noticeable when looking into water at an angle. Figure 3 shows the effect of refraction.
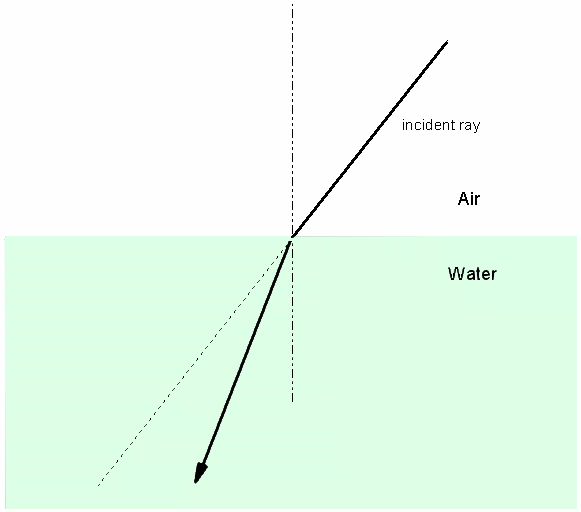 Figure 3: Refraction of light as it moves from a medium of one density to a medium of another.
Figure 3: Refraction of light as it moves from a medium of one density to a medium of another.
With a refractometer, light is shone through a very small amount of the sample at an angle and the degree of refraction, based on the density of the sample, influences the position of a line on a scale. The scale indicates the specific gravity of the sample. Figure 4 shows an example of the display in a refractometer.

Figure 4: View through the lens of a refractometer for a sample of seawater with a specific gravity of 1.027 (d20/20).
Finally, salinity can be measured directly via the conductivity of the water using a salinometer. This will give the most accurate measure of the salinity.
Which is the best method?
There are a number of criteria that can be used to assess the "best" method for measuring the specific gravity or salinity of seawater in our aquaria.
Precision, accuracy and reliability tend to come at a price. Precision relates to the level to which the number can be expressed; the number of significant digits. Accuracy is how close the readings are to the actual value for the sample being measured. Reliability relates to how repeatable the results are.
A good quality salinometer will be by far the most precise, accurate and reliable, but also the most expensive. Cheaper salinometers may give sufficient accuracy and reliability for aquarium use. Refractometers have a precision to 1 part in 70 (Pilson, 1998) which means a reading equivalent to 35ppt will indicate the sample is between 34.75 and 35.25. Refractometers are also accurate and reliable. Swinging arm hydrometer are easy to read and have higher precision than floating glass hydrometers, but they tend not to be very accurate and need to be calibrated to determine any necessary correction factor. Good quality floating glass hydrometers tend to be quite accurate but do not have a lot of precision.
In terms of ease of use, a refractometer is probably the easiest. Only one or two drops of sample are required to get a reading and the reading is easy to take. The fact that very little of the sample is required means that it is easy to test water in the bag with a newly acquired inhabitant and this information can be used to ensure the inhabitant gets suitable acclimation. Salinometers are also convenient and can be used as a monitor so the current salinity is always available. Salinometers do not require much of the sample, but do require more than a refractometer. Swinging arm hydrometers are easy to use, but require 50-100 mL of sample. Floating glass hydrometers are probably the least convenient to use. Ideally, the sample should be placed in a tall cylinder, such as a measuring cylinder and the hydrometer placed in that.
The temperature of the sample does not largely affect readings using salinometers or refractometers. Swinging arm hydrometers are claimed to be temperature compensating but this does not appear to be the case. The readings from floating glass hydrometers must be adjusted if the sample temperature is different from the calibrated temperature of the hydrometer.
A cheap floating glass hydrometer would be the cheapest to purchase, with many being less than $20. Of course, they tend not to be very accurate or precise, but once calibrated should give consistent results. Swinging arm hydrometers are reasonably cheap ($30-60). A good quality floating glass hydrometer will cost $50-150. A refractometer costs between $100 and $200. Cheap salinometers start at around $100 but you can pay thousands of dollars for a quality one.
Overall, a refractometer is probably the best purchase as you get a mix of ease of use, precision, accuracy and reliability for a reasonable price. When considering how much you are prepared to pay for a device to measure the specific gravity or salinity, don't forget about the value of the aquarium and livestock in it. Compared to the money tied up in the aquarium, spending $200 on a refractometer is not very much as a one off expense.
What is the correct specific gravity of water in a reef tank?
Before outlining the correct specific gravity of water for a reef tank, it should be noted that we should be aiming for the correct salinity and the specific gravity is simply the method chosen to estimate the salinity. So the first question should be, What is the correct salinity for a reef tank?
While there are variations in salinity of surface seawater from ocean to ocean (Atlantic is more saline than Pacific) and from location to location, the salinity is generally fairly constant, especially in reef areas. It can be assumed that the salinity of reef water is very close to S=35 and reef aquariums should be kept as close as possible to this value.
There is absolutely no reason to keep a reef tank at a salinity anything other than the natural salinity for the reefs from which the organisms were collected. Far too many hobby books recommend salinities (and specific gravities) that are significantly lower than natural reefs and I have yet to see any present a valid reason to do this.
While fish can generally tolerate salinities lower than that of natural seawater, many invertebrates cannot. Most marine invertebrates are osmoconformers and the osmotic concentration of their internal fluids are equal to that of the surrounding water (Schmidt-Nielsen, 1975) . Some of these osmoconformers also have solute concentrations identical or similar to their environment. Others actively regulate some solutes and maintain environmental concentrations of others. When these organisms are placed in water with a lower salinity, any non-regulated solutes will be reduced internally. This may cause some problems for the metabolism of the organism. Regulated solutes may also be a problem as the organism will have to work harder to maintain the correct concentrations. Lower than normal salinities will cause stress to the animals and are likely to result in reduced lifespan.
It is worth noting that the salinity of the Red Sea is somewhat higher (up to S=40) than that of Indo-Pacific reefs. If you are keeping any Red Sea organisms you should consider maintaining the tank at the salinity of the Red Sea. An aquarium with a mix of Red Sea and Indo-Pacific organisms may not be a good idea, particularly if it contains invertebrates from both regions. Within Australia this is not likely to be a problem as there are no Red Sea invertebrates available. If you are keeping Red Sea fish with Indo-Pacific organisms, it would be best to keep the salinity/specific gravity towards the high end of the range.
What is the specific gravity of seawater with a salinity of 35? As outlined above, this will depend on the standard using for specific gravity. Table 1 lists various specific gravity values for seawater (S=35) for various standards. Keeping the specific gravity 0.001 on either side of the value equivalent to S=35 would be the best.
Table 1: Approximate specific gravity of seawater (S=35) for various standards
| Standard | Specific Gravity | Range |
| d4/4 | 1.0278 | 1.027-1.029 |
| d15.6/15.6 | 1.0269 | 1.026-1.028 |
| d20/20 | 1.0266 | 1.026-1.028 |
| d25/25 | 1.0264 | 1.025-1.028 |
| d15.6/4 | 1.0259 | 1.025-1.027 |
| d20/4 | 1.0248 | 1.024-1.026 |
| d25/4 | 1.0234 | 1.022-1.025 |
In order to determine the correct specific gravity, you will need to know the standard your hydrometer or refractometer uses and for what temperature it is calibrated. Corrections to the reading may be required if the water temperature is different from the calibrated temperature of the meter.
For more information of appropriate specific gravity ranges for reef tanks, see What are Natural Reef Salinities and Temperatures...Really...and Does It Matter? by Dr Ron Shimek.
Some hydrometers and refractometers may not indicate the standard by which they are measuring the specific gravity. Others may not be accurate. Do not despair. If you have access to natural ocean seawater you can use this to calibrate your hydrometer or refractometer.
Maintaining and correcting the specific gravity of an aquarium
Maintaining the specific gravity in aquarium water is fairly simple and straightforward. All evaporated water should be replaced with freshwater (or an equivalent such as Kalkwasser) and should be added daily to reduce swings in salinity/specific gravity. Small variations in specific gravity are not problematic and are natural occurrences on reefs. No more than plus or minus 0.001 in one day is recommended. Adding 2L of fresh water to a 200L tank will only vary the specific gravity by 1% (around 0.00025). Small amounts of saline water removed by the skimmer or splashing can be corrected with water changes.
If the specific gravity of the aquarium is wrong, it should be changed gradually. If it is too low, it can be gradually raised by replacing evaporated water with seawater until the correct specific gravity is achieved. If the specific gravity is too high, small changes can be made simply by doing water changes with water at slightly lower than the correct specific gravity.
For larger changes, tank water can be removed and replaced with freshwater. Changes of no more than 0.001 to the specific gravity per day should be made. For example, to reduce the specific gravity from 1.030 to 1.029, 3% of the volume of tank water should be removed and replaced with fresh water. In a 200L tank this would equate to 6L. The fresh water should be added gradually to sump to ensure it is well mixed before returning to the tank.
Further reading
Specific Gravity: Oh How Complicated! by Randy Holmes-Farley.
References
Pilson M.E.Q. 1998. An Introduction to the Chemistry of the Sea. Prentice-Hall, Inc, Upper Saddle River, NJ.. 431pp.
Schmidt-Nielsen K. 1975. Animal Physiology: Adaptation and Environment. Cambridge University Press, London. 699pp.
Last updated: December 6, 2002